Research
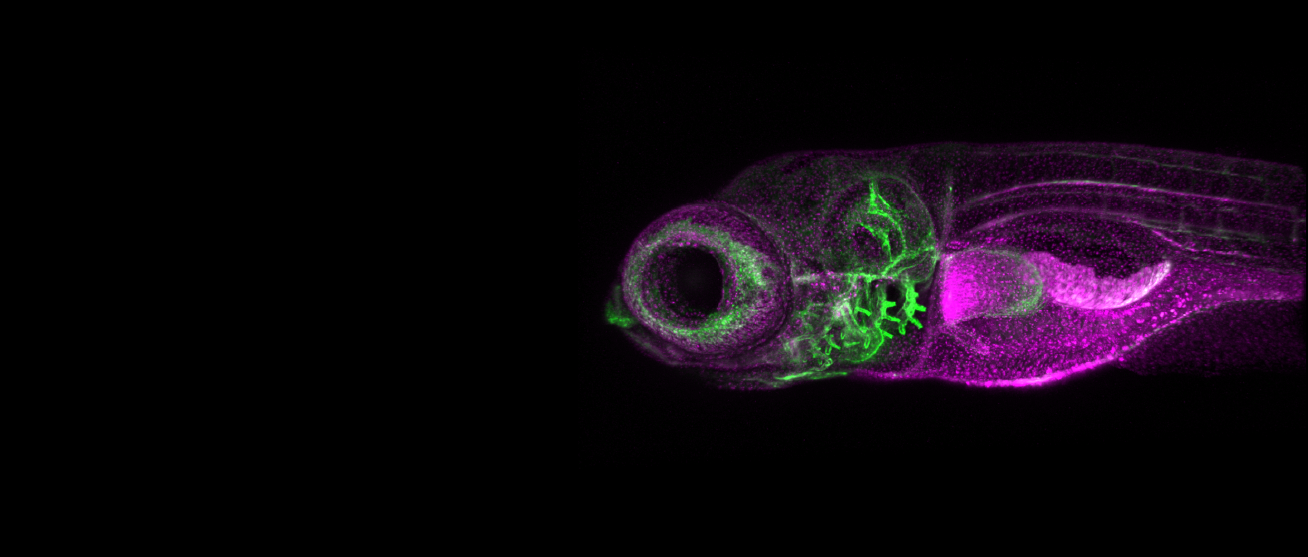
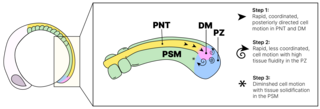
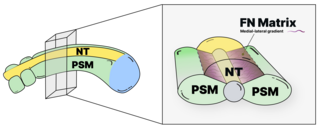
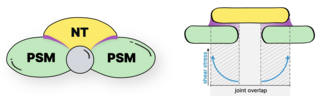
We study the systems morphogenesis of embryonic development at the molecular, cell, and tissue level. More specifically, we seek to uncover how molecular interactions drive cell behavior, how cell behavior shapes tissue properties, and ultimately, how the tissue properties guide embryo development. To answer these questions, we quantitatively study the process of early spinal column development in zebrafish by combining in vivo biophysics, embryology, genetics, and live imaging. Our experimental approach is driven by the idea that quantitative in vivo analysis will lead to fundamental insights into the emergence of biological organization from the collective interaction of its constituent parts.